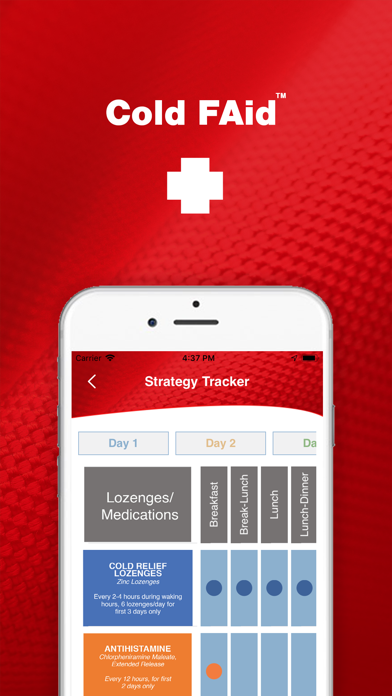
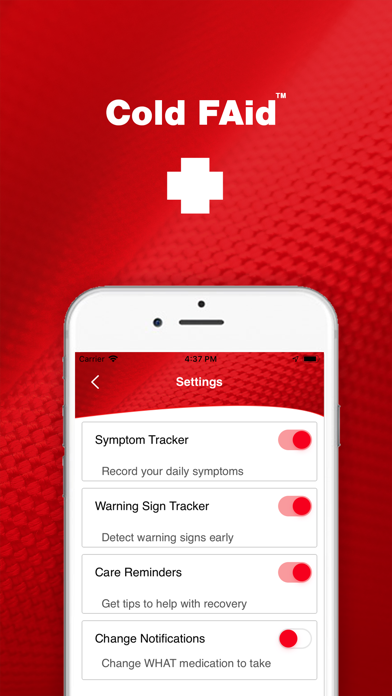
Partnering with the Cold FAid kit, this app is key to managing recovery from the common cold. This includes a research-based strategy regimen, notifications of WHAT medications to take WHEN, record of symptoms and medications, and knowledge of when/where to get medical/pharmaceutical care.
Disclaimer
Your use of the Service is at your sole risk. TheService is provided on an "AS IS" and "AS AVAILABLE" basis. The Service is provided without warranties of any kind, whether express or implied, including, but not limited to, implied warranties of merchantability, fitness for a particular purpose, non-infringement or course of performance.
Cold FAid its subsidiaries, affiliates, and its licensors do not warrant that a) the Service will function uninterrupted, secure or available at any particular time or location; b) any errors or defects will be corrected; c) the Service is free of viruses or other harmfulcomponents; or d) the results of using the Service will meet your requirements.
Medical Disclaimer
Cold FAid does not provide medical advice, diagnosis, or treatment. The contents of the Cold FAid app (text, infographics, photos, and other materials) provide information only. This content is not to be used in lieu of professional medical advice, diagnosis or treatment. For questions concerning a medical condition, always pursue the advice of your professional medical provider and do not delay seeking medical care due to anything you have read on the Cold FAid site. In the case of a medical emergency, call your professional medical provider or 911 immediately. Use of or dependence on any information provided by Cold FAid is solely at your discretion and risk.
The homeopathic product used in this kit is classified as a drug under the Food, Drug and Cosmetic Act. Over-the-counter (OTC) homeopathic drugs are reviewed for homeopathic efficacy, toxicology, adverse effects and clinical use by the Homeopathic Pharmacopoeia Convention of the United States (HPCUS), not the Food and Drug Administration (FDA). OTC homeopathic drugs comply with FDA requirements for labeling and Good Manufacturing Practices. Manufacturers must register with the FDA, are subject to FDA inspections, and must report adverse effects to the FDA. Compliance with HPCUS and the FDA does not guarantee safety, effectiveness or correct branding.